
■ -ΔE means the atom lost energy and now in more stable state ■ n = energy states (n=1,2.) | Z = atomic number (Z=1 for H) n 2 f = ∞ 2 if e - is removed from atom.Predicting deviations from ideal bond angles Writing the Lewis structures for a molecule with resonance Predicting whether molecules are polar or nonpolar Identifying a molecule from its electrostatic potential map Predicting and naming the shape of molecules with a central atom
Predicting the arrangement of electron groups around the central atom of a molecule Using the AXE notation to describe a molecule with a central atomĭeciding whether a Lewis structure satisfies the octet rule Predicting the compound formed by two main group elements Predicting the relative lattice energy of binary ionic compounds Predicting the single-bonded molecular compounds formed by two elements Predicting the relative stability of ionic crystals from a sketchĬalculating the heat of reaction from bond energies and Lewis structuresĬounting electron pairs in a Lewis structure with double or triple bondsĭrawing Lewis structures for simple organic compounds Naming the shape of molecules with one central atom and no octet-rule exceptions Writing Lewis structures for an expanded valence shell central atom Predicting bond angles in molecules with one central atom and no octet-rule exceptions Understanding how electrostatic energy scales with charge and separation Predicting the relative length and energy of chemical bonds Predicting the relative electronegativities of atoms Writing Lewis structures for diatomic molecules Understanding the definitions of ionization energy and electron affinityĬalculating the wavelength of a spectral line from an energy diagramĬalculating the wavelength of a line in the spectrum of hydrogen Identifying the electron added or removed to form an ion from an s or p block atom Interpreting an outer electron box diagram Understanding periodic trends in atomic size Interpreting the electron configuration of an atom or atomic ion in noble-gas notationĭeducing valence electron configuration from trends in successive ionization energiesĭeducing the block of an element from an electron configuration Identifying elements with a similar valence electron configuration
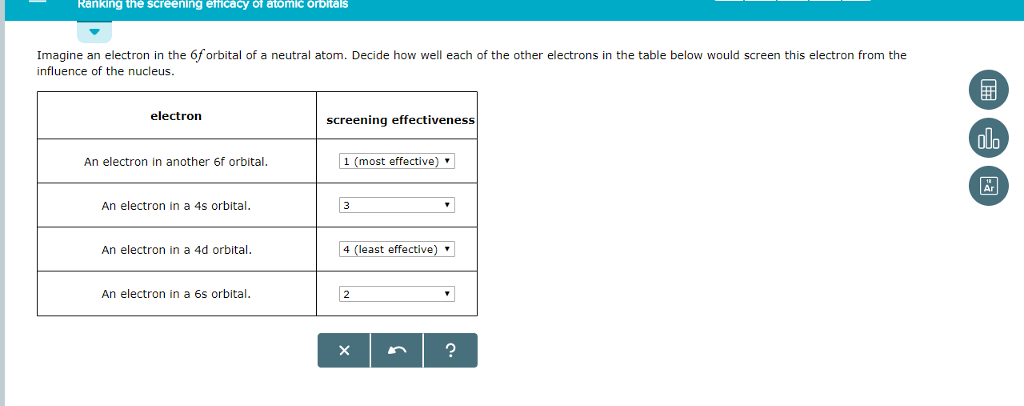
#RANKING THE SCREENING EFFICACY OF ATOMIC ORBITALS SERIES#
Writing the electron configuration of a first transition series atom Understanding the meaning of a de Broglie wavelength Understanding periodic trends in effective nuclear charge Predicting the qualitative features of a line spectrum Understanding periodic trends in atomic ionizability Interpreting the electron configuration of an atom or atomic ion Ranking the screening efficacy of atomic orbitalsĬalculating the capacity of electron subshells Interpreting the radial probability distribution of an orbital Knowing the subshells of an electron shellĬounting the electron shells in a neutral atom Interpreting the angular probability distribution of an orbitalĭeducing the allowed quantum numbers of an atomic electronĭrawing a box diagram of the electron configuration of an atom Predicting the relative ionization energy of elements Understanding the organization of the electromagnetic spectrum Interpreting the electron configuration of a neutral atom in noble-gas notationĭeciding the relative energy of electron shells Writing the electron configuration of an atom Interpreting the electron configuration of a neutral atom CHEM 1A CH 13 & 14 NOTES TABLE OF CONTENTS


 0 kommentar(er)
0 kommentar(er)
